Viewpoint on an article in Aljazeera (April 11th, 2023) on CRISPR
The cover picture shows Victoria Gray who was treated in 2019 as the first patient in the US with a CRISPR-Cas based therapy against sickle cell disease (SCD). She participated in a clinical trial and was treated with her own “repaired” blood stem cells. She was completely healed. Four years later, in 2023 she was the star at the international summit on human genome editing and told her story.
Interestingly, this was the same conference series where He Jiankui presented the internationally criticized birth of the germ line edited twins Nana and Lulu in 2018.
The article in Al Jazeera is undoubtedly good. It is necessary to discuss the ethical and practical issues of CRISPR therapies, but I believe that the discussion should be more complete.
Germline therapy was mostly banned even before the highly disturbing experiments by He Jiankui. “Mostly” means, that even the very strict “Ethics Council” in Germany does not exclude germ line therapy in general, but argues that it might be tolerable in very specific cases. But is this really an issue? The vast majority (about 99%) of genetic diseases can be avoided by in-vitro fertilization and embryo selection (PID – pre-implantation diagnostics). According to Mendelian genetics, at least 25% of the embryos will not be affected by the disease or do not carry the mutated gene at all (there are some very rare exceptions to this rule).
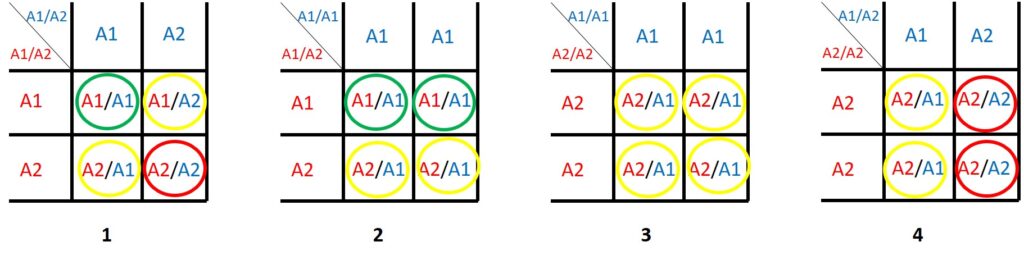
The figure shows the Mendelian inheritance of a mutated “disease gene”:
A certain gene A comes in two variants A1, which is normal and A2, which causes a disease or malfunction. Humans have two copies of each gene in every cell, so a person can be A1/A1 or A1/A2 or A2/A2. When they have two copies of the same variant, this is called homozygous, when they have different variants (like A1/A2) this is called heterozygous.
Depending on the gene, people with A2/A2 could be not viable or so sick that they cannot reproduce. A1/A2 could be viable but have a serious health problem.
For other genes, only A2/A2 has a serious problem and an A1/A2 person appears completely healthy. However, A1/A2 can give the “bad” gene variant to their children.
Germ cells (egg and sperm) only have one copy of each gene. A person with A1/A2 produces the same number of eggs (or sperm) with A1 or A2 while a person with A1/A1 will only produce A1 sperm (or eggs).
The squares above show different possible combinations of parents.
The green circles indicate offspring that is absolutely healthy, the red circles offspring that is for certain affected by the disease or not even viable. Depending on the gene, the yellow circles may represent persons that are completely healthy or may be affected by the disease.
Note that even when both parents are affected by the disease (square 1) they may have a 25% chance to have a child that is completely healthy!
When an A2/A2 (square 3 and 4) parent is sufficiently healthy to live and to reproduce, it is very likely that their A2/A1 offspring is mostly healthy.
Genes causing a disease are rare. Therefore the probablility that in a couple both partners carry the same “disease gene” is rather low. People who are homozygous for such a gene variant are (depending on the gene) often disabled, infertile or have a low life expectancy. It is very rare that a couple meets where both partners are homozygous for such a gene variant and desire to have children.
So, in most cases, some genetically healthy embryos are produced and can be easily selected by PID. The risk and burden for mother and child is negligible compared to a CRISPR therapy. Even the number of discarded embryos is lower with PID. Germ line therapy does not really have significant importance for avoiding human suffering. It is only “interesting” for human enhancement, i.e. implanting genes or changings genes, which “improve” an embryo and which are not present in the parents genome. But this is a completely different story and should not be discussed in the context of “eradicating human diseases”.
Somatic therapy on the other hand, has shown breathtaking advances in only a few years. However, costs for development and the therapy itself are extremely high. The scientific know-how and the required equipment are limited worldwide. Without a substantial further breakthrough, somatic therapy will not be affordable and technically feasible for the vast majority of patients. Ironically, those who are rich enough to afford a CRISPR therapy, serve as “guineapigs” for the less wealthy to improve the technology and investigate potential long-term effects. In the long run, this may also reduce the price tag.
With the very small number of patients being treated with CRISPR (approx. 100 so far) it is not meaningful to discuss issues of ethnicity and equity at this point. The vast majority of sickle cell anemia and ß-thalassemia patients has been treated and will be treated in technically advanced countries. However, the vast majority will be of Sub-Saharan and (eastern) Mediterranean origin because both diseases are prevalent in these regions.
In contrast to vaccines, CRISPR therapies cannot be simply shipped as a drug in a box. We will need advanced infrastructure and specialized scientists and medical doctors to implement these therapies in areas, where they are needed the most. Unfortunately, insufficient medical infrastructure correlates closely with the prevalence of sickle cell disease (and other serious health problems!).
For the sake of people living in these regions we have to support medical competence in general and not so much raise hopes for sophisticated therapies that are a long way down the road for the general application. For various reasons it will take many years and lots of efforts to establish sufficiently advance medical standards worldwide to meet even the basic needs of people.
It has to be noted, that somatic gene therapy is by no means the “magic bullet” to treat all heritable diseases. Even though there is considerable success for blood borne diseases, others are much more difficult to target.
In no way this means to reduce the efforts in advancing CRISPR therapies! There is an extremely high potential and without further research, clinical trials and approved applications (no matter how expensive they are), we will not achieve further breakthroughs and eventually get methods that are available for all.
Author: Wolfgang Nellen, BioWissKomm

